Traumatic brain injury (TBI) is usually caused by car accident, fall, contact movement or attack. In the US alone, the annual incidence rate of TBI is as high as 3 million 500 thousand. Currently, 5 million people have TBI related disability, which costs about 80 billion US dollars annually.
At present, the treatment of TBI mainly focuses on stabilizing the patients condition and alleviating symptoms, and there is no specific drug for the pathophysiological process of driving neurodegeneration after brain injury. TBI also significantly increased the risk of Alzheimers disease (AD). This suggests that there may be a common pathological mechanism between the two, and the emerging evidence points to S-nitrosylation and acetylation.
In fact, a recent small autopsy study reported increased acetylation of tau at lysine (k) 280 in the brains of three patients with AD and three patients with chronic traumatic encephalopathy. In a separate study, the same increase in tau acetylation at K280 was recorded in 10 AD patients, 5 patients with cortical basal degeneration, and 5 patients with progressive supranuclear palsy. In addition, in more than ten patients with severe AD, researchers found tau acetylation at k274 and k281.
However, these studies did not establish the driving force or pathological significance of the results. Given the interrelationship between TBI and ad, researchers are trying to determine whether the increase of tau acetylation is a causal pathophysiological factor in TBI, from which it is a potential convergence point. If so, can this provide an experimental platform to understand the pathophysiology related to tau acetylation in the brain.
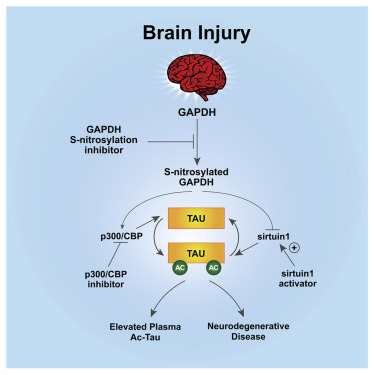
Recently, researchers reported in cell magazine that TBI can induce tau acetylation (AC tau), which also exists in human brains with AD. This is mediated by S-nitrosylated GAPDH, which can inactivate sirtun1 deacetylase and activate p300/cbp acetyltransferase simultaneously, and increase tau acetylation in neurons. The dislocation of tau protein leads to neurodegeneration and neurobehavioral disorder, as well as the accumulation of acetylated tau in blood.
Blocking S-nitrosylation of GAPDH, inhibiting p300 / CBP, or activating sirtuin1 can reduce the neurodegeneration, neurobehavioral disorder, and the accumulation of acetylated tau in blood and brain of TBI mice.
In AD patients with a history of TBI, the increase of acetylated tau protein in the brain will be further enhanced. Patients receiving p300 / CBP inhibitors salsalate or diflunisal show a lower incidence of AD and clinical diagnosis of TBI.
Therefore, tau protein acetylation is a therapeutic target and potential blood biomarker of TBI, which may represent the pathological fusion between TBI and AD.
Learn more about Abebio products:
Cindy